Experiment
An experiment is a study in which a treatment, procedure, or program is intentionally introduced and a result or outcome is observed (https://ori.hhs.gov/content/module-2-research-design-section-2#experimental-studies). Key characteristics are:
-Random assignments
-Control over extraneous variables
-Manipulate of the treatment conditions
-Outcome measurements
-Group comparisons
-Threats to validity
The most important of these characteristics are manipulation and control. In addition, experiments involve highly controlled and systematic procedures in an effort to minimize error and bias, which also increases our confidence that the manipulation "caused" the outcome.
Quasi-experiment
A quasi-experiment is an empirical study used to estimate the causal impact of an intervention on its target population without random assignment to treatment or control.

Randomized controlled trial (RCT)
Randomized controlled trial is a type of scientific experiment which aims to reduce bias when testing a new treatment. The participants in the trial are randomly assigned to either the group receiving treatment under investigation or to the control group. The control may be a standard practice, a placebo, or no intervention at all. It is the most rigorous way of determining whether a cause-effect relation exists between treatment and outcome. The RCT is considered the gold standard for a clinical trial.
One of the key feature is "randomization" to the group. All the participants have the same chance of being assigned to each of the study groups. Importantly, the characteristics of the participants are likely to be similar across the groups at the start of the comparison. This is intended to ensure that all potential confounding factors are divided equally among the groups that will be later compared.
RCTs are "controlled" so that researchers can reasonably expect any effects to be the result of the treatment or intervention, observing and comparing effects in the control group which not given the treatment or intervention.
Bias is avoided not only by randomization but also by blinding. When the groups that have been randomly selected from a population do not know whether they are in the control group or experimental group. The study is called "single blind". In "double blind" the researchers also do not know which participants are in the control group or the experimental group.
Intention to treat (ITT) is a strategy for the analysis of RCTs that compares patients in group which they were originally randomly assigned. All participants who were enrolled and randomly allocated to treatment are included in the analysis and are analyzed in the groups to which they were randomized. Inclusion occurs regardless of deviations that may happen after randomization, such as protocol violations, adherence to treatment protocol, dropout/withdrawals from the study. ITT provides 1) a more realistic estimate of average treatment effects in the real situation as it is normal that some participants may dropout or deviation from the treatment in every day practice and 2) helps to preserve the integrity of randomization process. ITT is a good approach of RCTs, but it will be problem if there is high dropout rate and poor adherence of the study. Reporting of any deviations from random assignment and missing response is essential of an ITT approach, as emphasized in the CONSORT guidelines on the reporting of RCTs.
Per protocol analysis is a comparison of treatment groups that includes only those participants who complete the treatment originally allocated. The results of per protocol analysis usually provide a lower of evidence but better reflect the effects of treatment. It can reduce the under- or overestimation of true effect which found in ITT. If done per protocol analysis alone, the analysis will be bias. Both intention to treat and per protocol analysis is recommended to report.
Per protocol analysis is a comparison of treatment groups that includes only those participants who complete the treatment originally allocated. The results of per protocol analysis usually provide a lower of evidence but better reflect the effects of treatment. It can reduce the under- or overestimation of true effect which found in ITT. If done per protocol analysis alone, the analysis will be bias. Both intention to treat and per protocol analysis is recommended to report.

Complex interventions are made up of many components that act both on their own and in conjunction with each other. There is no clear boundary between simple and complex interventions, but the number of components and range of effects may vary widely. Complex interventions are widely used in the health service, in public health practice, and in areas of social policy that have important health consequences, such as education, transport, and housing. The property of the intervention and the context into which an intervention is placed is important. Complex interventions may work better if tailored to local context than being completely standardized.
In 2000, the Medical Research Council (MRC) of the United Kingdom published a guideline to help researchers and research funders to recognize and adopt appropriate methods,and updated as this link. This BMJ paper summarized the issues that prompted the revision and the key massage of the new guidance. This figure is the key elements of the development and evaluation process.
In 2000, the Medical Research Council (MRC) of the United Kingdom published a guideline to help researchers and research funders to recognize and adopt appropriate methods,and updated as this link. This BMJ paper summarized the issues that prompted the revision and the key massage of the new guidance. This figure is the key elements of the development and evaluation process.

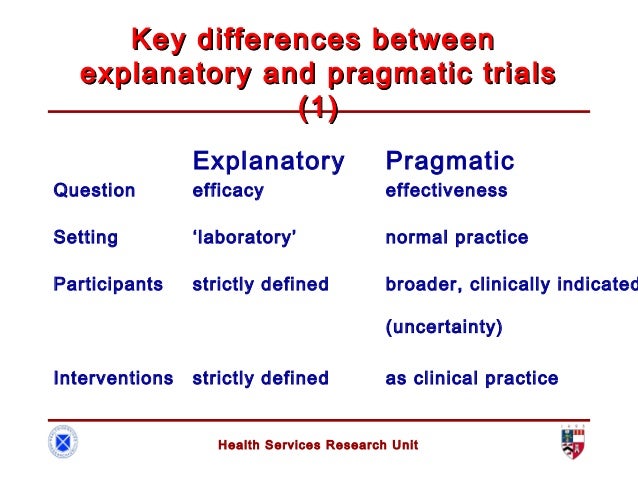
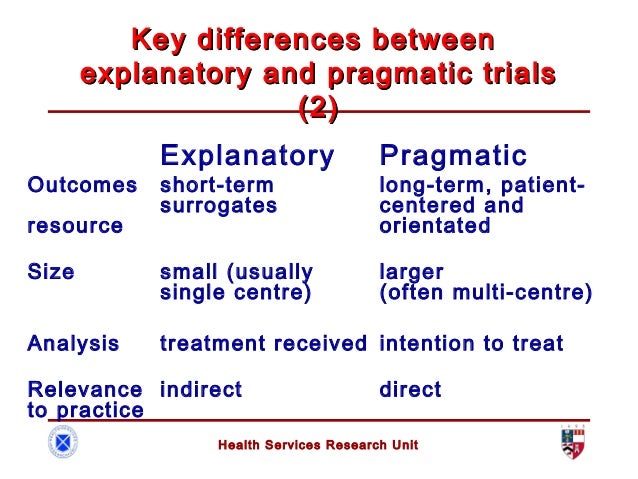
Pragmatic trials
Clinical trials have been the main tool used to test and evaluate interventions. Trials are either explanatory or pragmatic. Explanatory trials aim to test whether an intervention works under optimal situations. Pragmatic trials are designed to evaluate the effectiveness of interventions in real-word practice settings
In pragmatical trials, internal validity (accuracy of the results) and external validity (generalizability of the results) must be achieved, and must be prospectively registered and reported fully according to the pragmatic trials extension of the CONSORT statement.
No comments:
Post a Comment